Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O
Katano, Yoshio*; Aruga, Takeo; Yamamoto, Shunya; Nakazawa, Tetsuya; Yamaki, Daiju; Noda, Kenji
Journal of Nuclear Materials, 283-287(Part.2), p.942 - 946, 2000/12
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)no abstracts in English
*; *; *
JNC TJ8400 2000-018, 79 Pages, 2000/02
As a basic research for geological disposal of high-level radioactive wastes, diffusion behavior of radionuclides and corrosion behavior of overpack materials in clay buffer materials (bentonite) were studied. In the study on the diffusion behavior of radionuclides, basal spacing and water content were determined for water saturated, compacted Na-montmorillonite that is major clay mineral of bentonite. The apparent diffusion coefficients of Na , Sr
, Sr , Cs
, Cs and Cl
and Cl ions and their activation energies were also determined at different dry densities of montmorillonite. For all kinds of ions, the activation energies were found to increase as the dry density increased. These findings suggest that the diffusion mechanism of ions in compacted montmorillonite changed with increasing dry density. As a reasonable explanation for the changes in the activation energy, we proposed a multiprocess diffusion model, in which predominant diffusion process is considered to change from pore water diffusion to interlayer diffusion via surface diffusion when the dry density increases. The Na-montmorillonite is expected to alter by the ion exchange with Ca
ions and their activation energies were also determined at different dry densities of montmorillonite. For all kinds of ions, the activation energies were found to increase as the dry density increased. These findings suggest that the diffusion mechanism of ions in compacted montmorillonite changed with increasing dry density. As a reasonable explanation for the changes in the activation energy, we proposed a multiprocess diffusion model, in which predominant diffusion process is considered to change from pore water diffusion to interlayer diffusion via surface diffusion when the dry density increases. The Na-montmorillonite is expected to alter by the ion exchange with Ca ions, which could be introduced from groundwater and/or cementitious materials in a repository. The apparent diffusion coefficients of Na
ions, which could be introduced from groundwater and/or cementitious materials in a repository. The apparent diffusion coefficients of Na and Cs
and Cs ions and their activation energies were studied for Na/Ca montmorillonite mixtures in order to know the effect of this kind of alteration on the diffusion behavior of ions. It was found that the alteration of montmorillonite affected diffusion coefficients and the activation energies for both kinds of cations. These effects cannot be explained only by the pore water diffusion. The multiprocess diffusion model proposed in this study is suggested as the most reasonable explanation for the effects. The oxidation behavior of pyrite in bentonite during drying process was studied for understanding corrosion behavior of overpack materials in bentonite. There ...
ions and their activation energies were studied for Na/Ca montmorillonite mixtures in order to know the effect of this kind of alteration on the diffusion behavior of ions. It was found that the alteration of montmorillonite affected diffusion coefficients and the activation energies for both kinds of cations. These effects cannot be explained only by the pore water diffusion. The multiprocess diffusion model proposed in this study is suggested as the most reasonable explanation for the effects. The oxidation behavior of pyrite in bentonite during drying process was studied for understanding corrosion behavior of overpack materials in bentonite. There ...
; ; Sato, Haruo; Shibata, Masahiro
JNC TN8400 99-088, 58 Pages, 1999/06
Sorption and diffusion behavior of palladium, which has been identified as one of the hazardous radionuclides in performance assessment of HLW disposal, in bentonite, granodiorite and tuff was studied in order to make reliable data set for the performance assessment. Sorption experiments of Pd on bentonite, granodiorite and tuff were conducted as functions of pH, ionic strength and liquid to solid ratio by batch method under aerobic conditions at room temperature. The distribution coefficients (K ) of Pd on these solids were almost in the range of 10
) of Pd on these solids were almost in the range of 10 to 10
to 10 m
m /kg and were in the order of bentonite
/kg and were in the order of bentonite  granodiorite
granodiorite 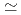 tuff. The sorption trends with change in PH, ionic strength and liquid to solid ratio are very similar between three solids. The K
tuff. The sorption trends with change in PH, ionic strength and liquid to solid ratio are very similar between three solids. The K values were the highest at pH5 and decreased with increasing pH between 5 and 11. The effect of ionic strength on K
values were the highest at pH5 and decreased with increasing pH between 5 and 11. The effect of ionic strength on K was not found in a range of 10
was not found in a range of 10 to 10
to 10 , but K
, but K values increased with increasing liquid to solid ratio. The width of variation in K
values increased with increasing liquid to solid ratio. The width of variation in K was one order of magnitude in a liquid to solid ratio of 0.1 to 1 m
was one order of magnitude in a liquid to solid ratio of 0.1 to 1 m /kg. Sorption behavior of Pd is different from that of divalent metal ions such as Ni and Co etc. and chemical analogy may be inappropriate. The dominant aqueous species of Pd in the expermental conditions studied is estimated to be neutral species, Pd(OH)
/kg. Sorption behavior of Pd is different from that of divalent metal ions such as Ni and Co etc. and chemical analogy may be inappropriate. The dominant aqueous species of Pd in the expermental conditions studied is estimated to be neutral species, Pd(OH) (aq) by the thermodynamic calculations. The K
(aq) by the thermodynamic calculations. The K values of Pd on three solids were relatively high and uncharged complexes may be more strongly sorbed. The pH dependency of K
values of Pd on three solids were relatively high and uncharged complexes may be more strongly sorbed. The pH dependency of K values suggests that Pd sorption is most likely to be occurring onto positively charged S-OH
values suggests that Pd sorption is most likely to be occurring onto positively charged S-OH type site which are progressively removed (to from SOH and SO
type site which are progressively removed (to from SOH and SO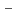 sites) at higher pH values. Diffusion behavior of Pd in bentonite was also studied by in-diffusion method as a function of dry density. The D
sites) at higher pH values. Diffusion behavior of Pd in bentonite was also studied by in-diffusion method as a function of dry density. The D values obtained based on the instantaneous planar source model were in the orders of ...
values obtained based on the instantaneous planar source model were in the orders of ...
Sato, Haruo; ; ; *; *; Yui, Mikazu
PNC TN8410 97-127, 57 Pages, 1997/08
Retardation of key nuclides is one of the most important mechanisms to be examined specifically and modelled for the performance assessment of geological disposal of radioactive waste. We have been studing diffusion of nuclides into the pore spaces of the rock matrix, sorption of nuclides on the rock pore surfaces and pore properties to quantify the degree of nuclide retardation in fractured crystalline rock. The work has concentrated on predominant water conducting fracture system in the host granodiorite in the Kamaishi In Situ Test Site, which consists of fracture fillings and altered granodiorite. Through-diffusion experiements to obtain effective and apparent diffusion coefficients (Da and De, respectively) for Na, Cs, HTO, Cl and Se as a function of ionic charge at 22  25
25 C and batch sorption experiments for Cs, Sr, Se,
C and batch sorption experiments for Cs, Sr, Se,  U and
U and  Pu were conducted on fracture fillings, altered and intact granodiorite. The experiments only for Se, a redox sensitive element, were done in an N2-atmospheric glove box (O
Pu were conducted on fracture fillings, altered and intact granodiorite. The experiments only for Se, a redox sensitive element, were done in an N2-atmospheric glove box (O
 1 ppm) to keep the chemical species. In situ groundwater (pH8.7
1 ppm) to keep the chemical species. In situ groundwater (pH8.7 9.5) sampled from the same place as rock samples was used for the experiments. Porosity and density of cach rock sample were determined by both water saturation method and mercury porosimetry, and pore-size distribution and specific surface area of pores were measured by mercury porosimetry. The porosity is in the order; fracture fillings (5.6%)
9.5) sampled from the same place as rock samples was used for the experiments. Porosity and density of cach rock sample were determined by both water saturation method and mercury porosimetry, and pore-size distribution and specific surface area of pores were measured by mercury porosimetry. The porosity is in the order; fracture fillings (5.6%)  altered rock (3.2%)
altered rock (3.2%)  intact rock (2.3%). The pore-size distribution of the intact and altered granodiorite is ranging from 10 nm to 0.2 mm, and the fracture fillings have that of 50 nm to 0.2 mm, but a lot of pores were found around 100 nm and 0.2 mm in the fracture fillings. The effective diffusion coefficients for all species (Na
intact rock (2.3%). The pore-size distribution of the intact and altered granodiorite is ranging from 10 nm to 0.2 mm, and the fracture fillings have that of 50 nm to 0.2 mm, but a lot of pores were found around 100 nm and 0.2 mm in the fracture fillings. The effective diffusion coefficients for all species (Na , Cs
, Cs , HTO, Cl
, HTO, Cl , Se0
, Se0
 ) are in the order of fracture fillings
) are in the order of fracture fillings  altered rock
altered rock  intact rock in proportion to these porosities. Effective diffusion ...
intact rock in proportion to these porosities. Effective diffusion ...
Kawato, Takaya*; Ishida, Keisuke*; Yamamoto, Takeshi*; Minato, Daisuke*; Fujisaki, Kiyoshi*; Hamamoto, Takafumi*; Mihara, Morihiro
no journal, ,
no abstracts in English